-
 EducationAs I was doing this project, the specter waiting for me as we started wrapping up our projects was the prospect of having to answer the question, “So what?” What is the point of this research? I spent most of my time working on the “methods,” the techniques (surgeries, soldering, coding) that became the experimental […]
EducationAs I was doing this project, the specter waiting for me as we started wrapping up our projects was the prospect of having to answer the question, “So what?” What is the point of this research? I spent most of my time working on the “methods,” the techniques (surgeries, soldering, coding) that became the experimental […] -
 FellowshipPennywise, the dancing clown The newest addition to our mantis shrimp family is a gorgeous green-black Gonodactylus smithii named Pennywise. The Gonodactylus genus has been my fourteen-year-old brother’s favorite genus ever since I told him that it essentially means scrotum fingers, as the two raptorial appendages held at the ready take on a somewhat humorous shape. […]
FellowshipPennywise, the dancing clown The newest addition to our mantis shrimp family is a gorgeous green-black Gonodactylus smithii named Pennywise. The Gonodactylus genus has been my fourteen-year-old brother’s favorite genus ever since I told him that it essentially means scrotum fingers, as the two raptorial appendages held at the ready take on a somewhat humorous shape. […] -
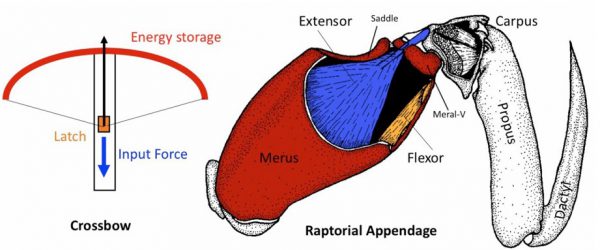 FellowshipI want you to do me a favor. I want you to hit me as hard as you can. What’s that? You want more background? Folks, things have started to pick up. Perhaps the most important development since June 11th has been the christening of our two mantis shrimp, which will give me an excuse to talk […]
FellowshipI want you to do me a favor. I want you to hit me as hard as you can. What’s that? You want more background? Folks, things have started to pick up. Perhaps the most important development since June 11th has been the christening of our two mantis shrimp, which will give me an excuse to talk […] -
 EducationHi folks! My name is Dan and I am a student at the University of Massachusetts Amherst studying neuroscience and minoring in computer science. Back at school, I work in a songbird lab where I listen to neurons fire in zebra finches, and I’m on the ballroom dance team. Outside of working, sleeping, and eating, […]
EducationHi folks! My name is Dan and I am a student at the University of Massachusetts Amherst studying neuroscience and minoring in computer science. Back at school, I work in a songbird lab where I listen to neurons fire in zebra finches, and I’m on the ballroom dance team. Outside of working, sleeping, and eating, […]