-
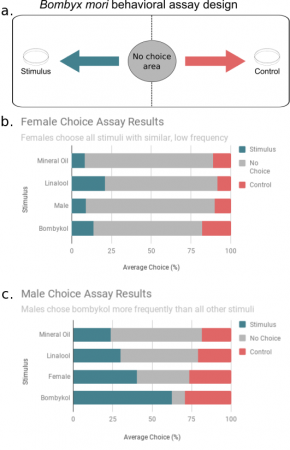 FellowshipHello, everyone! Welcome back to the last installment of silkmoth updates. Things are starting to wrap up here this summer, and I’ve begun to analyze the data I have been collecting. Behavioral Data Analysis Last post, I explained the behavior assay that I am using with the moths to demonstrate how the sex pheromone bombykol can alter […]
FellowshipHello, everyone! Welcome back to the last installment of silkmoth updates. Things are starting to wrap up here this summer, and I’ve begun to analyze the data I have been collecting. Behavioral Data Analysis Last post, I explained the behavior assay that I am using with the moths to demonstrate how the sex pheromone bombykol can alter […] -
 FellowshipHello, everyone! Jess here. Lots of exciting things have happened in the last two weeks. First, I have begun to raise a group of silkworms into moths (#mothmom). This involves feeding them Mulberry leaves from my backyard each day, keeping everything extremely clean and crossing my fingers in hopes that I know what I’m doing. […]
FellowshipHello, everyone! Jess here. Lots of exciting things have happened in the last two weeks. First, I have begun to raise a group of silkworms into moths (#mothmom). This involves feeding them Mulberry leaves from my backyard each day, keeping everything extremely clean and crossing my fingers in hopes that I know what I’m doing. […] -
 InternshipGreetings, this is Trevor coming live from Ann Arbor in a basement… We have one week left in the internship and things are finally starting to come together. Last time I made a post, I was without a doubt on the struggle bus in terms of getting data worthy of a poster, let alone a journal […]
InternshipGreetings, this is Trevor coming live from Ann Arbor in a basement… We have one week left in the internship and things are finally starting to come together. Last time I made a post, I was without a doubt on the struggle bus in terms of getting data worthy of a poster, let alone a journal […] -
 InternshipHey! What’s up? My name is Trevor Smith, currently a senior at the fabulous Michigan State University, and I am lucky enough to be participating in an internship at Backyard Brains this summer. I am currently working on pheromone detection in moth antennae, specifically how sensitive male moths antennae are to the female pheromone used […]
InternshipHey! What’s up? My name is Trevor Smith, currently a senior at the fabulous Michigan State University, and I am lucky enough to be participating in an internship at Backyard Brains this summer. I am currently working on pheromone detection in moth antennae, specifically how sensitive male moths antennae are to the female pheromone used […]