You know what’s great about fruit flies? Nothing.
fig. 1 Fruit flies suck
Nothing, that is, other than their benefit as a model organism for simple and fast transgenic experimentation — but who really cares about all that. Drosophila melanogaster are butts, so what if they could die? Well they can (vinegar and plastic-wrap), we don’t need science for that. We can go one further. What if we can make the flies loathe existence as much as the rest of all life hates them? What if we could take away the one thing that makes their nasty, brutish, and short existence bearable? Make them lose life’s purest love? The love of…sugar?
Yeah, we did that.
With
Look at this little guy, basking in the sweet, sweet 625nm rays of ghost sugar:
fig. 2 How long can you hold out against science little guy, how long?
If you remember from my first post, red light activates the Gr5a sweet taste neuron, making the fly feel like a kid in a candy store after the adult-apocalypse. Now if we interrupt this tiny love affair with a SYRINGE OF SCIENCE and also quinine we can get the fly to associate the bliss of sugar (which flies love), with the bitter sting of quinine (which flies do not love).
That’s classical conditioning, Kyle. After a few pairings of the two, the flies become depressed. Or I would, if I were a fly, because at that point they can’t stomach anything sweet, and don’t even respond to a fly sized glob of syrup. I’d do things to a human sized glob of syrup you’d probably try to get me arrested for, and the knowledge that science could some day deprive me of this pleasure is a sobering thought. After this I sat in a dark room for 3 days eating candy.
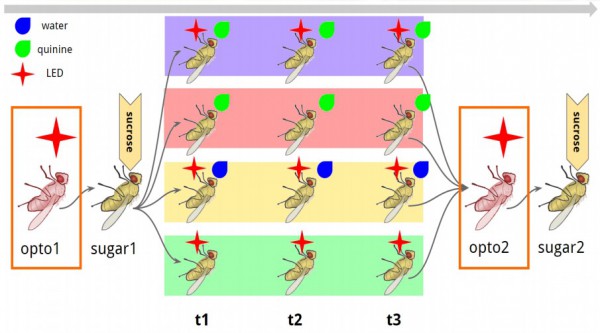
When I came out, the data was still there and I learned to love the bomb. Here’s a bit more detail on the way we played with fly tastes and the numbers we got:
fig. 3 What I done do to them flies
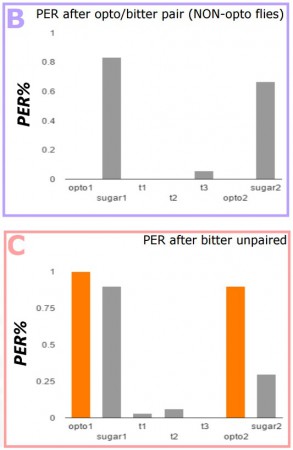
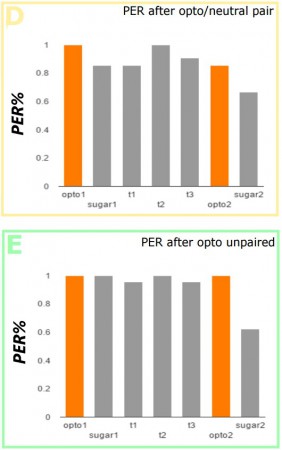
The flies were taken off of food for 12hr before experimentation, then adhered to a foil slide with nail polish and mounted on a stand. For the conditioning test (A, purple), proboscis extension reflex (PER) was first tested via optogenetic, and then actual sugar stimulation, to establish a baseline of both the light-induced and real sugar-induced response. For each trial, PER was optogenetically induced 3 times, and quinine applied to the extended proboscis. After 3 trials, PER was measured as a response to light, and then sugar again. A simple control used non-optogenetic flies-with the gene for the optogenetic channel, but no second gene activating its expression, and then the same opto/quinine pair trial (B, purple). For further control trials, (C, red) quinine was applied to the proboscis sans opto- stimulation, (D, yellow) opto- stimulation was paired with water, and (E, green) opto- stimulation was used without quinine. Though this may seem like a lot of controls experiments, we want to establish as firmly as possible that the response we are getting is solely a result of the paired conditioning experiment we are running.
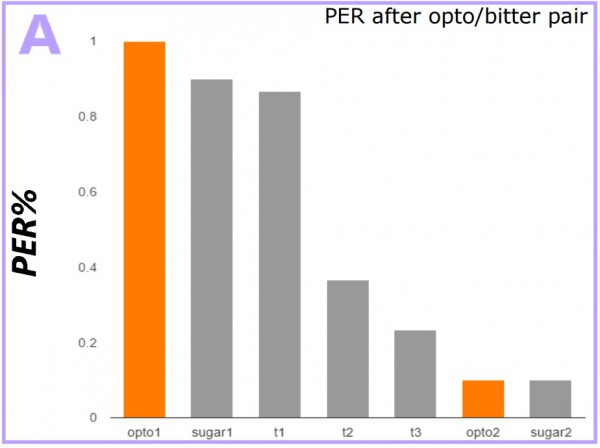
fig. 4 ALL THE DATA
These data show strong aversion to sugar after the conditioning. Only the trials where sweet taste activation is paired with bitter shows a marked decrease in response to sugar over the average indicating that the result was due to conditioning, not random chance. Future experimentation will also see pairing the light activation with a neutral taste to the flies, like salt, to see if we can condition the flies to respond to salt as they respond to sugar, as well as the possibility of optogenetically activated bitter taste activation combined with the introduction of real sugar. Optogenetics is the cutting edge of neuroscience technology, however, so whatever comes next will be exciting!