-
 EducationThe Backyard Brains 2018 Summer Research Fellowship is coming to a close, but not before we get some real-world scientific experience in! Our research fellows are nearing the end of their residency at the Backyard Brains lab, and they are about to begin their tenure as neuroscience advocates and Backyard Brains ambassadors. The fellows dropped in […]
EducationThe Backyard Brains 2018 Summer Research Fellowship is coming to a close, but not before we get some real-world scientific experience in! Our research fellows are nearing the end of their residency at the Backyard Brains lab, and they are about to begin their tenure as neuroscience advocates and Backyard Brains ambassadors. The fellows dropped in […] -
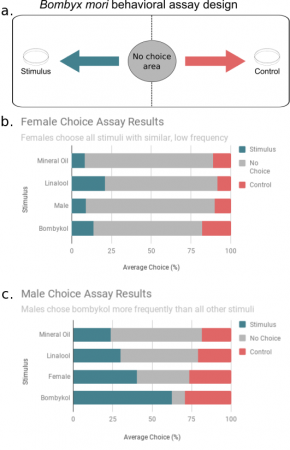 FellowshipHello, everyone! Welcome back to the last installment of silkmoth updates. Things are starting to wrap up here this summer, and I’ve begun to analyze the data I have been collecting. Behavioral Data Analysis Last post, I explained the behavior assay that I am using with the moths to demonstrate how the sex pheromone bombykol can alter […]
FellowshipHello, everyone! Welcome back to the last installment of silkmoth updates. Things are starting to wrap up here this summer, and I’ve begun to analyze the data I have been collecting. Behavioral Data Analysis Last post, I explained the behavior assay that I am using with the moths to demonstrate how the sex pheromone bombykol can alter […] -
 FellowshipHello, everyone! It’s Jess again. Since I last wrote, I have shifted all my work from cockroaches over to silk moths. I’ve had to make a few modifications to my protocols, but overall the transition has been smooth. Working with the silk moths has been far more enjoyable than the cockroaches (no offense roaches, but […]
FellowshipHello, everyone! It’s Jess again. Since I last wrote, I have shifted all my work from cockroaches over to silk moths. I’ve had to make a few modifications to my protocols, but overall the transition has been smooth. Working with the silk moths has been far more enjoyable than the cockroaches (no offense roaches, but […] -
 FellowshipOne of the most attractive things about a BYB Summer Fellowship is the chance to spend a summer in colorful Ann Arbor. We changed the program name from an internship to a fellowship because of the lasting connections made throughout the summer, and these connections are made possible by the things we all do together! […]
FellowshipOne of the most attractive things about a BYB Summer Fellowship is the chance to spend a summer in colorful Ann Arbor. We changed the program name from an internship to a fellowship because of the lasting connections made throughout the summer, and these connections are made possible by the things we all do together! […] -
 FellowshipFresh, organic, locally sourced meditation researchLast Friday, Backyard Brains once again opened our doors (even wider–they’re always open during business hours!) to our fellow and aspiring citizen scientists as a part of this year’s Ann Arbor Tech Trek! Dozens of local tech companies had their doors open to the public that evening and we, like […]
FellowshipFresh, organic, locally sourced meditation researchLast Friday, Backyard Brains once again opened our doors (even wider–they’re always open during business hours!) to our fellow and aspiring citizen scientists as a part of this year’s Ann Arbor Tech Trek! Dozens of local tech companies had their doors open to the public that evening and we, like […] -
 FellowshipHello, everyone! Jess here. Lots of exciting things have happened in the last two weeks. First, I have begun to raise a group of silkworms into moths (#mothmom). This involves feeding them Mulberry leaves from my backyard each day, keeping everything extremely clean and crossing my fingers in hopes that I know what I’m doing. […]
FellowshipHello, everyone! Jess here. Lots of exciting things have happened in the last two weeks. First, I have begun to raise a group of silkworms into moths (#mothmom). This involves feeding them Mulberry leaves from my backyard each day, keeping everything extremely clean and crossing my fingers in hopes that I know what I’m doing. […] -
 EducationHi all! I’m Jess. I’m a senior neuroscience major from Westminster College in Salt Lake City, UT. It’s a small, private liberal arts school you’ve probably never heard of, but I promise you it’s the best small, private liberal arts school you’ve probably never heard of (with an awesome neuroscience program). Prior to my academic […]
EducationHi all! I’m Jess. I’m a senior neuroscience major from Westminster College in Salt Lake City, UT. It’s a small, private liberal arts school you’ve probably never heard of, but I promise you it’s the best small, private liberal arts school you’ve probably never heard of (with an awesome neuroscience program). Prior to my academic […] -
 EducationFrom left: Ben, Anusha, Yifan, Jessica, Aaron, Jess, Greg Gage (not a Fellow), Maria, Dan, Anastasiya, Molly, Ilya Meet the Fellows, See the Projects The fellows are off to a great start! This week has been focused on them getting their feet wet with our kits and learning about what we do here at Backyard Brains. Be […]
EducationFrom left: Ben, Anusha, Yifan, Jessica, Aaron, Jess, Greg Gage (not a Fellow), Maria, Dan, Anastasiya, Molly, Ilya Meet the Fellows, See the Projects The fellows are off to a great start! This week has been focused on them getting their feet wet with our kits and learning about what we do here at Backyard Brains. Be […] -
 InternshipGreetings, this is Trevor coming live from Ann Arbor in a basement… We have one week left in the internship and things are finally starting to come together. Last time I made a post, I was without a doubt on the struggle bus in terms of getting data worthy of a poster, let alone a journal […]
InternshipGreetings, this is Trevor coming live from Ann Arbor in a basement… We have one week left in the internship and things are finally starting to come together. Last time I made a post, I was without a doubt on the struggle bus in terms of getting data worthy of a poster, let alone a journal […] -
 InternshipHey! What’s up? My name is Trevor Smith, currently a senior at the fabulous Michigan State University, and I am lucky enough to be participating in an internship at Backyard Brains this summer. I am currently working on pheromone detection in moth antennae, specifically how sensitive male moths antennae are to the female pheromone used […]
InternshipHey! What’s up? My name is Trevor Smith, currently a senior at the fabulous Michigan State University, and I am lucky enough to be participating in an internship at Backyard Brains this summer. I am currently working on pheromone detection in moth antennae, specifically how sensitive male moths antennae are to the female pheromone used […]